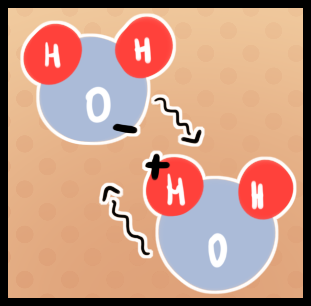
What makes water bond? Water is polar, this is what helps it bond. Polarity means that the water has both a positive and negative charge on it. Polarity has also made water be:
- A universal Solvent
- Adhesive
- Cohesive
- Less dense when frozen
- Neutral (pH of 7)
- High Heat Capacity
- And more :)
The properties of water is what helps make it different to other liquids
Universal Solvent: Since water is polar(charged), it can disolve other polar molecules, such as salt.
Less dense when frozen: Solid water floats in liquids. Most other solids sink.


